In recent years, there has been an obvious trend of innovation in the vaccine market. mRNA vaccine has become an emerging vaccine technology route. The basic principle is to introduce mRNA expressing antigen targets into the body through a specific delivery system, express the protein in the body and stimulate the body to produce specific immune responses, including humoral immunity and cellular immunity. The vaccinated person can obtain high-efficiency immune protection.
In layman terms, mRNA is like a computer program, a synthetic virus that can be programmed to carry and deliver DNA-encoded instructions that tell cells which proteins to make.
Compared with traditional vaccines, mRNA vaccines have the highest protection efficacy of 94% to 95%.
▎mRNA: From technical reserves to accelerated verification
There is a high technical threshold for mRNA vaccines. Only Moderna and Pfizer/BioNTech's new coronavirus mRNA vaccine varieties have been approved for marketing. According to the 2021 financial report data released by Pfizer, the revenue of the mRNA new crown vaccine Comirnaty reached a record $36.781 billion, which can be described as a well-deserved "King of Medicine".
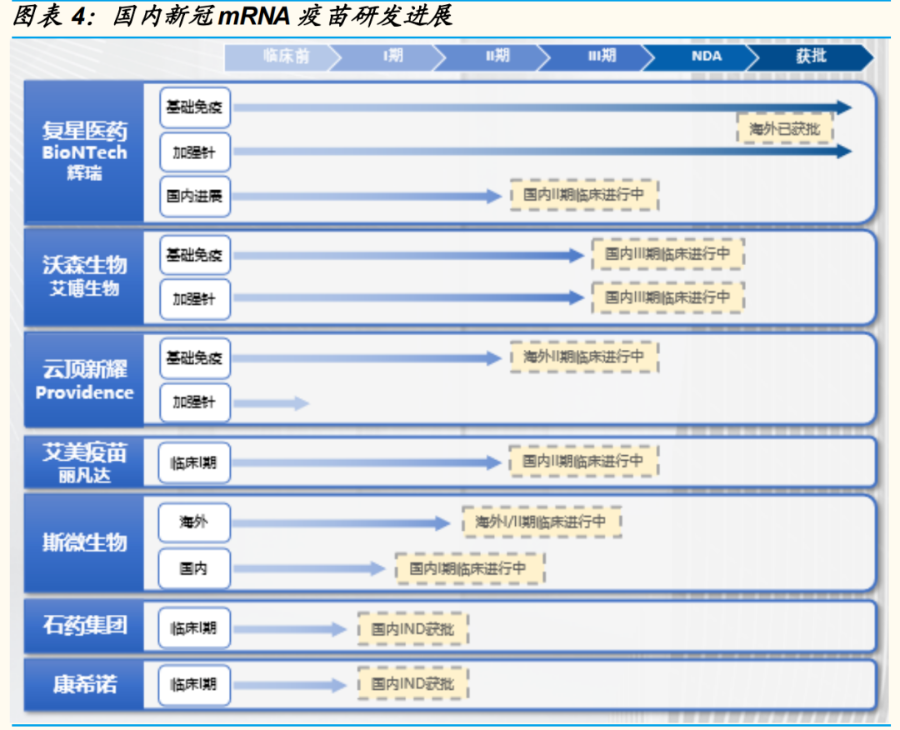
This has also stimulated the nerves of mRNA vaccine developers at home and abroad. Recently, two more mRNA vaccines in China have been announced to enter the clinical stage.
CSPC announced that its mRNA new crown vaccine SYS6006 has been approved for clinical use . Preclinical studies have shown that SYS6006 has good immune protection efficacy against the current mainstream mutant strains including Omicron and Delta, can provide immune protection to the body through humoral and cellular immunity, and can generate memory B cells to provide long-term effects protective effect.
CanSino Biologics announced that its mRNA new crown vaccine has been approved for clinical use. The results of preclinical studies show that the vaccine can induce high titers of neutralizing antibodies against a variety of important variants identified by the World Health Organization (including the current circulating strain), which is comparable to the existing new crown vaccine developed based on the prototype strain. Stronger than broad-spectrum and more effective in protecting the body from infection by existing variants.
In addition to the companies mentioned above, the domestic distribution of mRNA new crown vaccines include Sinopharm China Bio-Funuojian, Fosun Pharma, Lanque Bio, Genting Xinyao, Zhifei Bio (cooperating with Shenxin Bio), Kangtai Bio (cooperating with Jia Jia) Chenxihai cooperation), Ruike Bio (cooperation with Regis Bio), Thick Storage Nano, etc.
Some people may want to ask, now that China has vaccinated the new crown vaccine more than one billion times, and the mRNA new crown vaccine is still in the clinical stage. Is there no market demand after it is launched?
▎How big is the market space for mRNA vaccines?
①Market space: According to the estimation of WenXie et al.'s article published in NatureReview in September 2021, it is estimated that the global mRNA preventive vaccine market size in 2035 will be US$12-15 billion, and the market size of personalized tumor vaccines will be about US$7-10 billion. The market size of mRNA therapy (protein replacement therapy, etc.) is about 4 billion to 5 billion US dollars, and the total market space of mRNA in the field of treatment and prevention is about 23 billion to 30 billion US dollars.
②Pipeline composition: The prevention and treatment pipelines of leading companies account for about half of each, and tumor vaccines and protein replacement white therapy in the therapeutic field account for a higher proportion. In the "Global Leading Report Series 3: Debate on mRNA Technology and Platform" published on August 19, 2021, the basic characteristics of mRNA technology in the fields of sequence design, delivery system, and large-scale production were analyzed. The core of Biotech's evolution It is a technical barrier and a commercialization cooperation network. The marginal impact of the clinical approval of two domestic mRNA new crown vaccines on the industry is the acceleration of process platform verification and the acceleration of new vaccine/therapeutic research and development. We are optimistic about the domestic technology platform Biotech from technological catch-up to corner overtaking .
As of April 8 , 2022 , 64.7 % of the world's population has received at least one dose of the new crown vaccine, but only 14.8% of the population in low-income countries has received at least one dose of the new crown vaccine, and the global dose of the new crown vaccine booster dose per 100 people is 21.7 doses, We believe that there is still room for initial immunization in low-income countries in the world. It is assumed that there are 800 million people in low-income countries in the world (estimated by the United Nations in 2019 ) , and 60% of them are vaccinated against the new crown . According to the new crown vaccine export space for low-income countries, the export space is 18 billion to 20 billion yuan.
▎In addition to the new crown, what other application scenarios are there for mRNA vaccines?
In fact, most pharmaceutical companies prefer to call themselves "mRNA vaccines". The new crown vaccine is just the tip of the iceberg in the application of mRNA technology. It can also be used in many fields, such as tumors, monoclonal antibodies and protein drugs. Substitution, immunodeficiency related diseases, heart failure, rare diseases, assisted reproductive process and medical aesthetics, etc.
Compared with traditional vaccines, the advantages of mRNA vaccines are more prominent. Not only are they fast in development and design, and have high efficiency against mutated viruses, but they can also induce strong immune responses, achieve rapid mass production, and have no risk of gene integration. Therefore, the application scenarios It is quite extensive, mainly including preventive vaccines (such as new coronavirus, RSV, CMV, HPV and other infectious diseases), therapeutic vaccines (such as personalized cancer vaccines, KRAS-mutated lung/colorectal cancer and other tumors), both of which account for all mRNAs The proportions of pipelines are 42% and 18%, respectively.
The "14th Five-Year Plan" for the development of the pharmaceutical industry clearly points out that it is necessary to keep up with the development trend of vaccine technology, support the construction of mRNA vaccines, and improve the guarantee level of the vaccine supply chain. With the continuous advancement of domestic mRNA vaccine research and development, the demand for upstream supply chains continues to increase, and the demand for mRNA vaccines and related products in the upstream supply chain is optimistic.
It can be seen that mRNA vaccines have become the direction that pharmaceutical companies are scrambling to deploy .
The article is an analysis point of view of Jingtai and does not constitute investment advice. Please read it carefully.





